Do not miss out the two solar heat webinars in March offered by the IEA Solar Heating and Cooling Programme together with partners. You can join the next IEA SHC […]
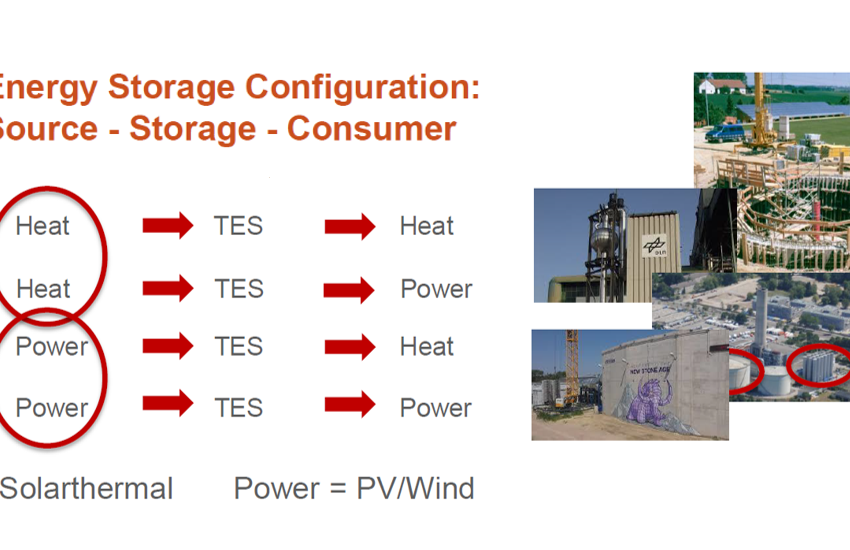
In his keynote speech at Eurosun 2020, Dr Andreas Hauer talked about the different roles that heat storage could play as part of a whole systems approach to energy policy. […]
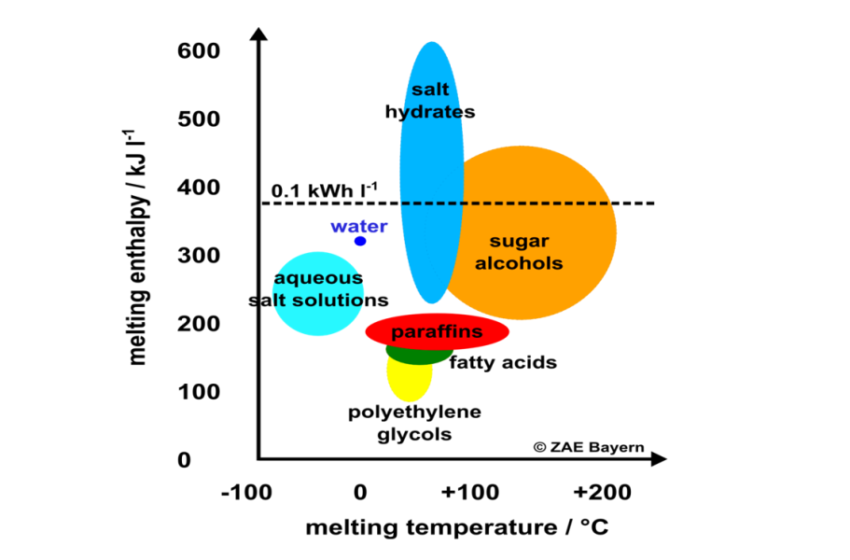
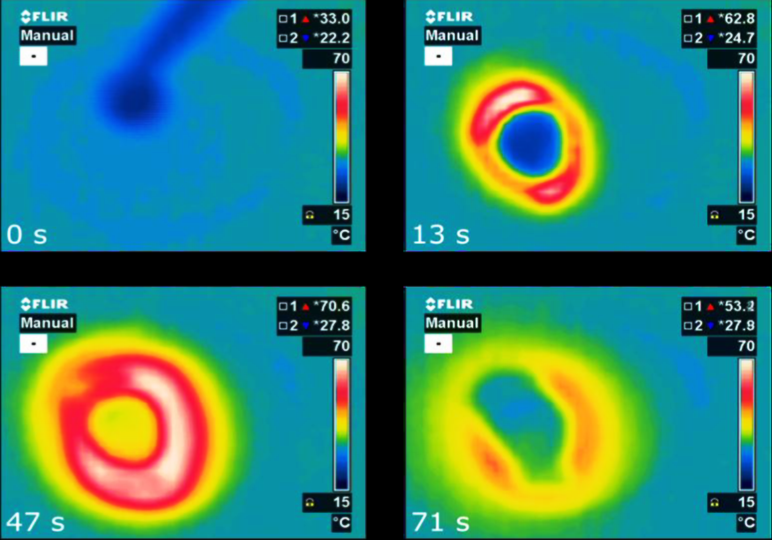
Salt hydrates are the most interesting phase change material in the low-temperature range of up to 150 °C. Their volume-related enthalpy at melting is much higher than the enthalpy of […]
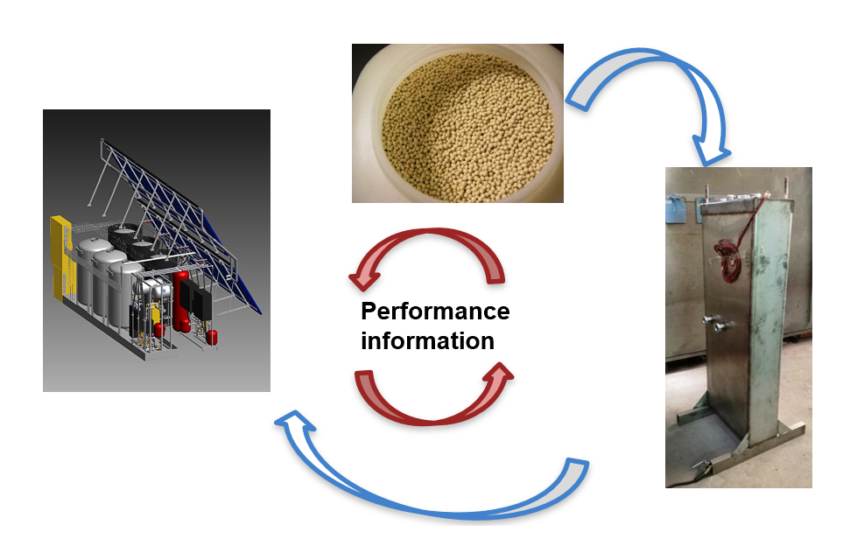
Research into compact thermal energy storage is made difficult by the fact that the performance of storage materials depends significantly on their intended purpose. For example, storage tanks that are […]
Research and industry experts in thermochemical storage materials are invited to participate in a workshop that will take place on 11 and 12 November in Erlangen, Germany. The objective of […]
Compared to water, phase change materials (PCMs) offer more capacity to store heat in tanks. As a consequence, researchers in all corners of the world have been developing new materials […]
Microporous hydrophilic substances such as zeolites are recognised as promising materials for very efficient energy storage. Solar thermal systems are used to dry the material and store all of the […]
Thermochemical materials (TCMs) used in storage tanks show higher heat capacities than water and smaller losses over time. They are crucial to an increase in solar use among consumers and […]